A fundamental aspect of ensuring patient safety and the quality of a drug is to conduct stability studies and stress tests. The stability, associated storage conditions and shelf life of an active pharmaceutical ingredient (API) or finished drug product (DP) are necessary parameters in the development of a new drug, through its approval and marketing. To support you in this extensive process, we offer you the complete range of services: from storage of your product to analysis, from planning, development and validation of analytical test methods, to complete documentation and evaluation of the collected data.
Thanks to our large number of cGMP qualified climate cabinets, we can carry out a wide variety of stability studies according to the storage conditions described in the ICH Guidelines (ICH Q1A/Q1F/VICH GL3), and thus based on the climate zones defined by the WHO (I-IV). Our range of services includes:
The storage removal intervals defined for the respective study design can be determined individually but are usually based on the guidelines and the minimum storage periods required for submission described therein. Storage within the framework of the maximum shelf life of up to five years is also possible.
In addition, we offer you the opportunity to carry out stress tests in order to identify degradation products at an early stage and to be able to make statements about the stability of a substance and suitable methods of analysis. In addition to thermal stress testing, by increased temperature (e.g. 60°C) or increased humidity (e.g. 90% rH), we can also test products for photostability as described in the ICH Q1B guideline.
Here, too, the conditions can be determined individually, but are usually based on the guidelines.
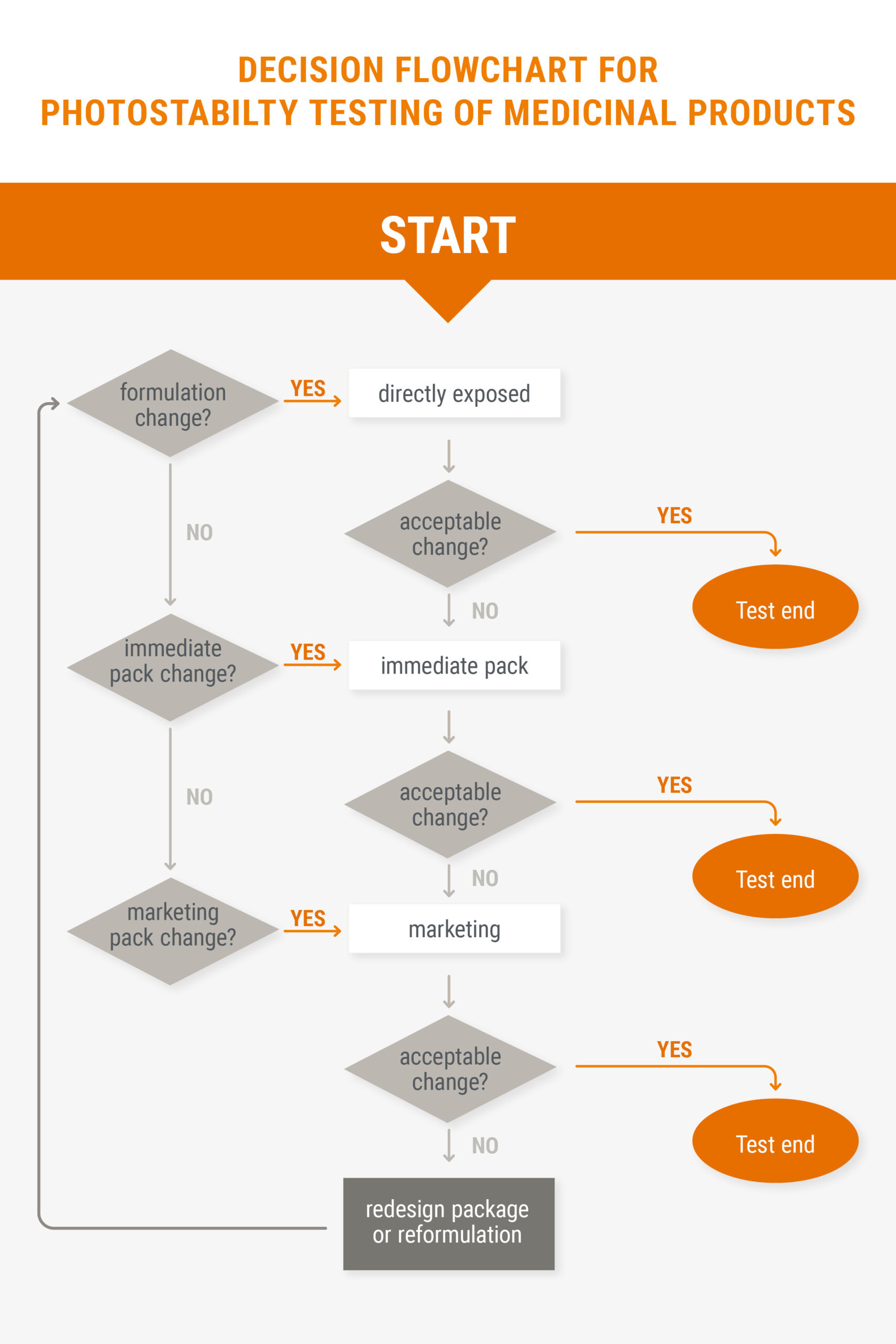
Figure: Flowchart for the photostability measurements of pharmaceutical products from the ICH Q1B guideline.
In order to be able to make a statement about the effects of short-term temperature fluctuations on the stability of a drug, freeze ad thaw studies can be carried out. In this case, the drug is stored for a short time at lower temperatures (down to -20°C) for e.g. 2 days, and then for a short time at higher temperatures (up to 40°C).
Since stability studies are long-term projects, data analysis can be carried out as part of the Accelerated Stability Assessment Program (ASAP) for faster evaluation. For this purpose, representative batches are exposed to more extreme temperature (10°C-70°C) and humidity (10%-90% rH) conditions for a period of up to one month and then analyzed. By statistical evaluation of the data obtained, among other things on the basis of the Arrhenius equation, information on storage stability, critical parameters and the expected shelf life can be predicated at an early stage. This does not replace the data required for submission of a stability study but makes it possible to obtain valid data on Critical Quality Attributes (CQA) of a drug in a shorter time and to contribute to the conception of Quality by design (QbD).
Depending on the properties of your product, be it an API or the finished DP, from solids to liquids, we can offer you a broad analytical portfolio. Of course, we are also happy to assist you in the conception of the analysis.
If you do not find the desired storage conditions in our standard portfolio, contact us and we will be happy to check our options for you.
Benefit from our many years of experience in conducting stability studies and our know-how across a wide range of analytics – please feel free to contact us!
